Calibration for:
Date:
Time:
Name & ID#:
1. ___ Be sure all equipment needed for calibration is present and operable.
2. ___ Note and record the air temperature in the calibration chamber. Also record the barometric pressure in the area.
Chamber Temp. K Atmospheric Pressure mmHg
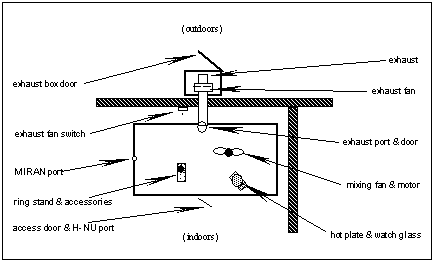
3. ___ Fasten the H-NU probe to the ring stand near the chamber center.
NOTE: During simultaneous H-NU/MIRAN calibrations, the H-NU should be placed on a chair near the front chamber door. The small hole near the edge of this door should then be used to allow H-NU probe access to the chamber.
4. ___ Turn on the H-NU to STANDBY, and allow five minutes for warm-up.
5. ___ To clear calibration chamber, open fan box(outdoors), exhaust port, and chamber front door; and run exhaust fan for ten minutes.
6. ___ Open doors and windows to help clear interfering factors from the lab air.
7. ___ Calculate volumes needed for solvent additions to the chamber. (See Appendix A of the H-NU/MIRAN calibration procedures)
Volume for 1/2 TLV: µl
Volume for TLV: µl
Volume for twice TLV: µl
8. ___ With H-NU on STANDBY, turn the ZERO knob until the response meter reads 0.
9. ___ Turn H-NU to 0-20, and record the meter response as background.
background _______
NOTE: This background reading should be subtracted from all subsequent readings.
10. ___ Turn off the exhaust fan, and close the exhaust port door.
11. ___ Close the chamber front door, and seal the probe access hole with tape.
12. ___ Remove a solvent from the yellow cabinet. Pour a small amount into a 30mL beaker and cover beaker with a watch glass to reduce evaporation.
13. ___ The first calibration point for a given factor on the H-NU should be done at the ACGIH threshold limit value (TLV) to find the proper span setting. Add to the chamber the amount of solvent (see Appendix A) that will evaporate into the TLV for that factor and turn on the hot plate and mixing fan until a stable reading is noted. Turn off the fan and hot plate. Turn the SPAN knob until the TLV or a factor of ten of the TLV is noted on the meter. (This may require adjustment of the RANGE selector.) Record the span setting and x-factor. (The x-factor is the number by which the meter-face reading must be multiplied to get the actual concentration.) Clear the chamber for 10 minutes, reseal the chamber, and proceed using the new span setting and x-factor.
New Span Setting:
New X-Factor:
14. ___ Add the amount of solvent (to the watch glass on the chamber hot plate) that will evaporate to one-half the TLV of the solvent in use.
15. ___ Initialize mixing fan and watch for the solvent to evaporate.
NOTE: Some solvents may require heating to evaporate entirely. (If necessary, provide for changes in chamber air temperature caused by the hot plate on the checklist.) Turn off the hot plate as the last bit of solvent evaporates.
New Chamber Temperature (if different from (1.), above): K
16. ___ Stop the chamber mixing fan after approximately five minutes.
17. ___ Record the meter reading.
FIRST READING:
SECOND READING:
THIRD READING:
NOTE: Record readings only when the needle remains relatively motionless for >15 seconds.
18. ___ Add the amount of solvent that will raise the solvent's chamber concentration to the TLV.
19. ___ Repeat steps 14-16.
20 ___ Add the amount of solvent that will raise the solvent's chamber concentration to twice the TLV.
21. ___ Repeat steps 14-16.
22. ___ Clear chamber for 10 minutes before starting the next calibration.
NOTE: Spend this time making a simple graph of your data, with actual chamber solvent concentration versus concentrations shown by the H-NU. Redo calibrations which yield grossly nonlinear plots. Plots for most solvents should be linear to ~300 ppm.
23. ___ When finished calibrating for a day, return the lab to the state in which you found it. Be sure to turn off hot plate and return equipment to proper areas.
jh/8-92